To Determine Density the Quantities That Must Be Measured Are
Its volume must be measured in a non-water-based liquid such as alcohol. One way to analyze the precision of measurements would be to determine the range or difference between the lowest and the highest measured values.
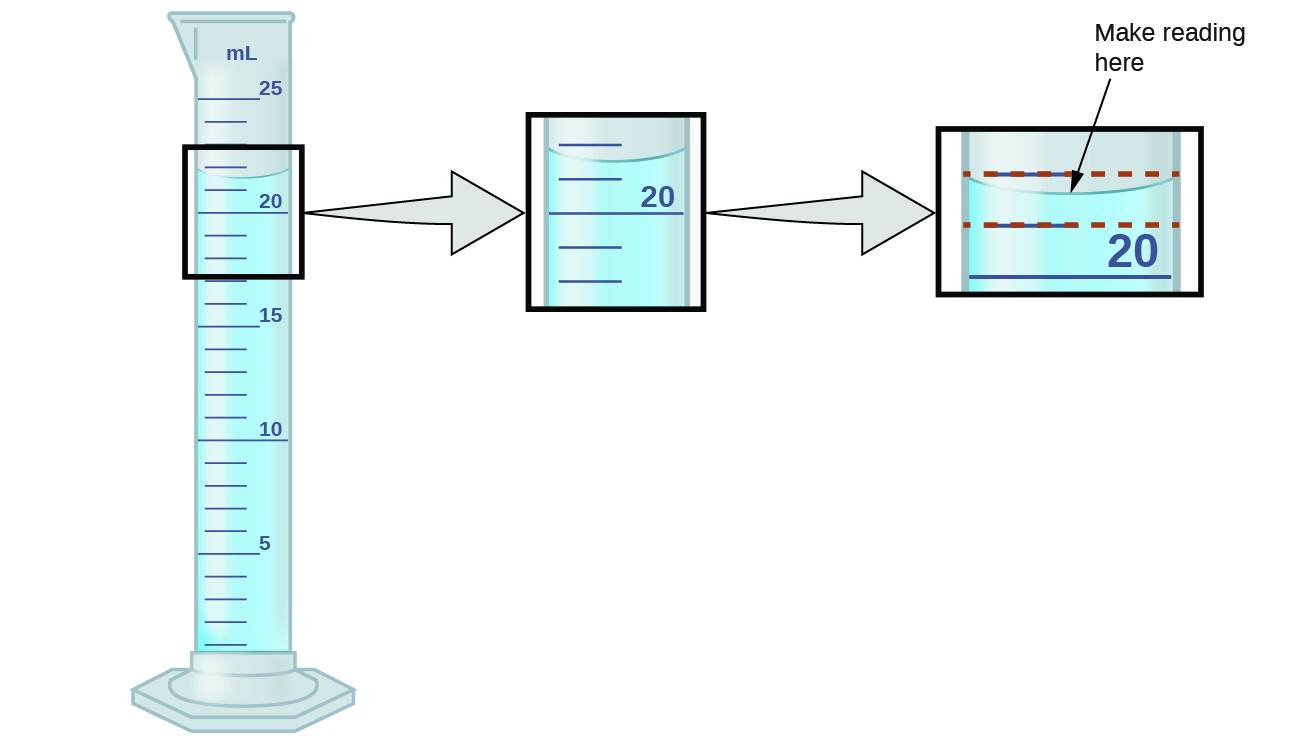
1 5 Measurement Uncertainty Accuracy And Precision Chemistry
Thus the measured values deviated from each other by at most 03 inches.

. 2386 Part 3 1963 or BS 812-21995. In the case of the printer paper measurements the lowest value was 109 inches and the highest value was 112 inches. Measuring liquid volumes is easy given the fact that liquids have no definite shape and will Search Powered by JRank Science Clarified Real-Life Chemistry Vol 3 - Physics Vol 1 Density and Volume eLearning Masters Online tamucedueLearning Master of Science from AM Commerce Apply.
Measurement helps us determine the amount of a given set of objects. The bulk density and void percentage of aggregate can be evaluated using standard test methods of applicable codes such as ASTM C 29C29M-17a IS. The portion of an x-ray beam that is absorbed depends on the penetrating ability of the radiation and the size and density of the body section exposed.
The uncertainty of the 2500mL pipet. The situation is more satisfactory for fibres and rods. Specific gravity for liquids is nearly always measured with respect to water at its densest at 4 C or 392 F.
The mass is normally measured with a scale or balance. To express this measurement we first need a quantity to associate everything we are measuring and these. The bulk density or unit weight is the weight per unit volume mass per.
Have paper towels to hand. Normal laboratory safety procedures should be followed. Consequently it must be concluded that an experimental approach remains the most accurate method of determining the packing density of particles.
The procedure provided in this article is based on the specification of ASTM standard ASTM C 29C29M-17a. Two aspects of the absorbed radiation energy must be. Other quantities Dimension.
Linear measurements typically generate a background signal that is observed in the absence of measurand and must be subtracted from the measured values. To determine the density of a solid and use this to determine further quantities. In most clinical situations more than 90 is absorbed.
For gases the reference is air at room temperature 20 C or 68 F. Our understanding of chemical processes thus depends on our ability to acquire accurate. In nuclear imaging procedures a large percentage of the energy emitted by radionuclides is absorbed in the body.
First it is observed that random packing densities of elongated particles are low when particle aspect ratios are high Nardin et al 1985. Relative density or specific gravity is the ratio of the density mass of a unit volume of a substance to the density of a given reference material. Similarly hydrostatic weighing uses the displacement of.
This background signal limits the sensitivity of the measurement and is. Barbie pegasusun sihri tek parça türkçe dublaj izle Not defteri lisans Eylül barbie pegasusun sihri izle. This measured value has a number and a unit associated with it for example 3 mathrmkg mangoes 1 mathrmkg tomatoes 500 mathrmg coriander etc.
The volume may be measured directly from the geometry of the object or by the displacement of a fluid. Use a falling-ball method to determine the viscosity of a liquid Objective To time the fall of a ball through washing-up liquid to determine the viscosity Safety Washing-up liquid spills are very slippery and must be cleared up at once. For example measured absorption is proportional to the concentration of the measurand as predicted by the Beer-Lambert law.
In particular assume that the density of the cone is uniform across cross sections parallel to its base but that in each such cross section that is a distance x units from the origin the density of the cross section is given by the function rhox 400 frac2001x2text measured in kgm3text Determine and evaluate a definite integral whose value is the mass of. The term relative density is often preferred in. Chemistry is the study of matter.
To determine the density of aluminum applying the technique of water displacement and use that value to determine the thickness of a piece of aluminum foil. To determine the density of a liquid or a gas a hydrometer a dasymeter or a Coriolis flow meter may be used respectively. For one density measurement you used the pipet to determine the volume and the analytical balance to determine mass.

Determining Densities Activity Teachengineering

Determining The Density Of A Solid And Liquid General Chemistry Jove

No comments for "To Determine Density the Quantities That Must Be Measured Are"
Post a Comment